About Me
I am a Principal Scientist within the External Collaboration and Experimentation (Experimental and Translational Medicine) area at Novo Nordisk. My expertise lies in quantitative evidence synthesis, data fusion, HTA and observational science, and in driving external engagement and collaboration. I am currently developing statistical and causal inference methods for the synthesis of evidence across different data sources. From December 2025, I have joined the Department of Statistical Science at University College London as an Honorary Research Fellow.
I have ample experience as a statistician, researcher and statistical consultant, providing support to the pharmaceutical, biotechnology, and healthcare consultancy sectors. Prior to my current role, I was a statistical methodologist at Novo Nordisk and the lead statistician for health technology assessment at Bayer Pharmaceuticals. Prior to that, I was an independent contractor providing statistical support to contract research organizations such as IQVIA and ICON plc, and public bodies such as SickKids.
See my Google Scholar profile for an updated list of publications.
- Biostatistics
- Causal Inference
- Evidence Synthesis
- Data Fusion
- Health Technology Assessment
PhD Statistical Science
University College London
MRes Financial Computing
University College London
MSc Machine Learning
University College London
BSc Mathematics and Physics
University of Bath
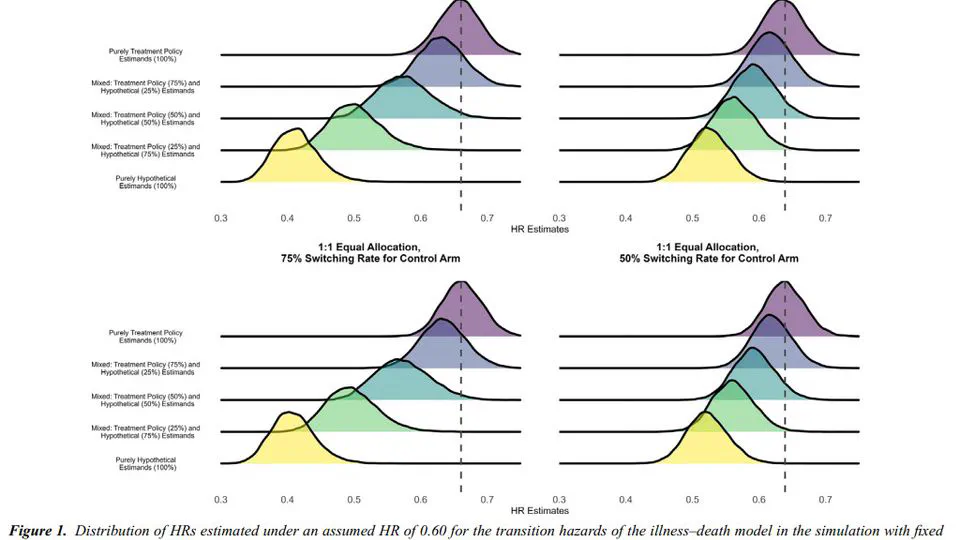
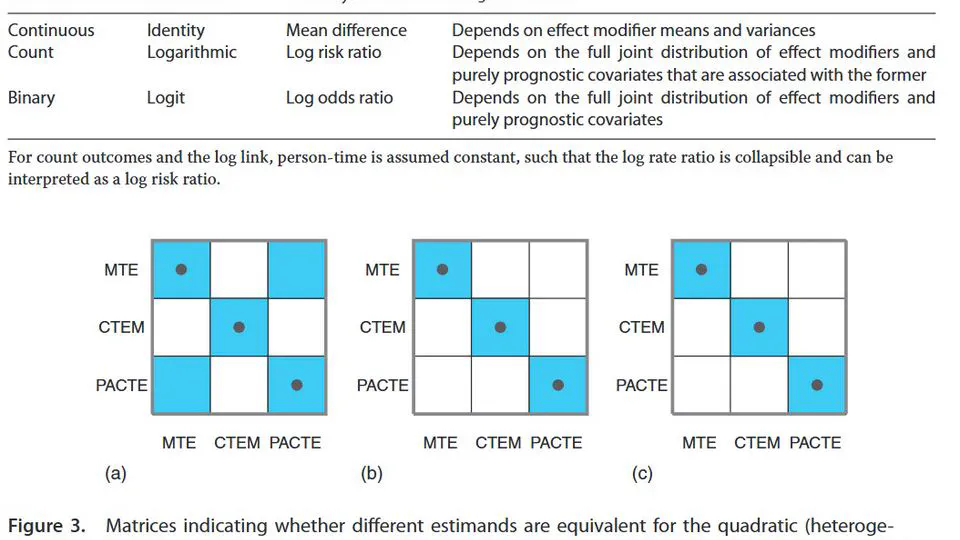
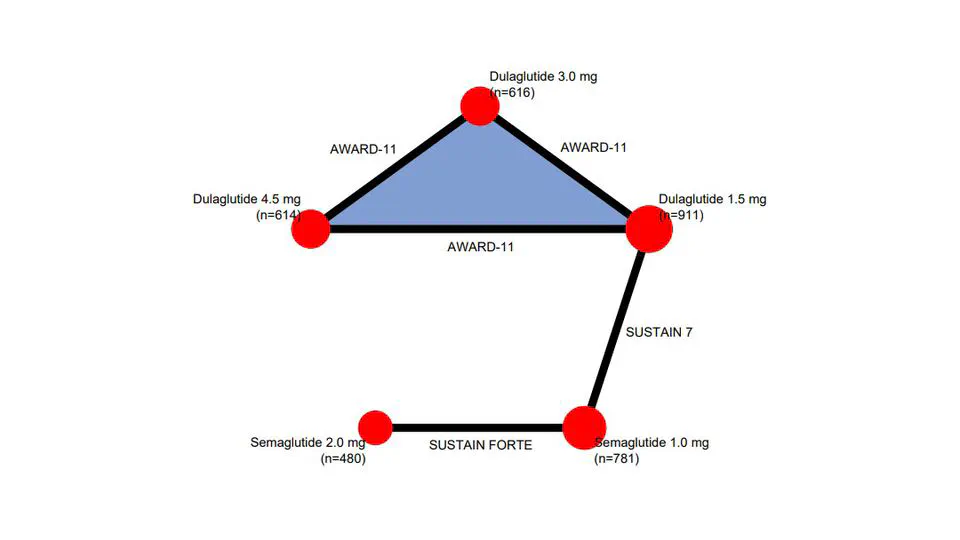
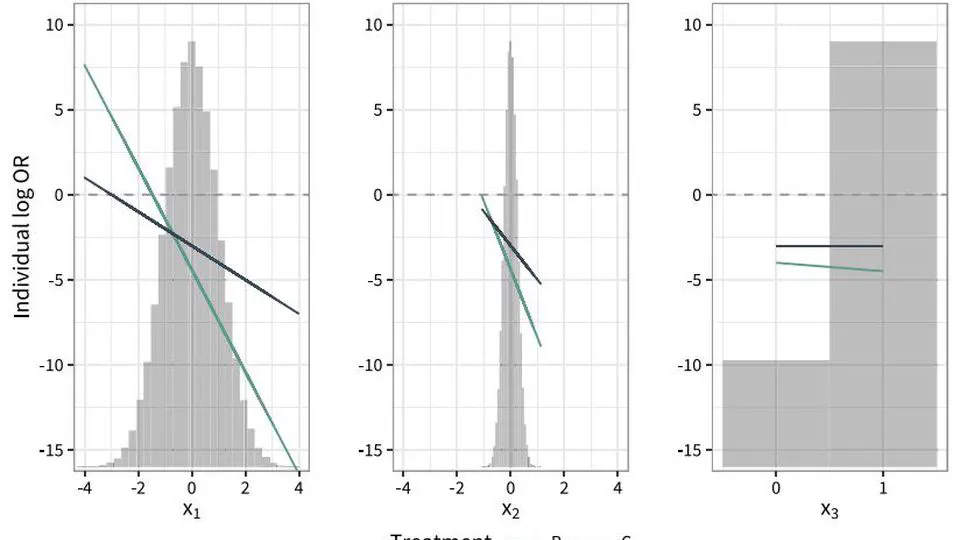
My research lies on the interface between statistics and health technology assessment, involving both methodological and applied problems. My primary interests are the development of statistical methodology to compare treatments in the absence of head-to-head clinical trials, adjusting for differences in patient populations and overcoming limited access to subject-level data.
Current interests: indirect treatment comparisons, covariate adjustment, transportability, estimands

JICI Lab Seminar Series
Dec 2, 2025
ISPOR Europe 2025
Nov 12, 2025

ISPOR Europe 2025
Nov 10, 2025

10th EFSPI Regulatory Statistics Workshop
Sep 10, 2025

46th Conference of the International Society for Clinical Biostatistics
Aug 25, 2025